When a company discovers that one of its medical devices is faulty, it should issue a recall to protect the public from any dangers the product poses. However, that does not necessarily mean everyone should immediately stop using the product. According to the U.S. Food and Drug Administration, it could mean the company needs to issue modified instructions or make repairs or adjustments to correct the defect.
The FDA conducts its own evaluation of the product’s defect to determine whether it creates a health hazard, and what steps everyone should take next.
(Article continues below infographic)
________
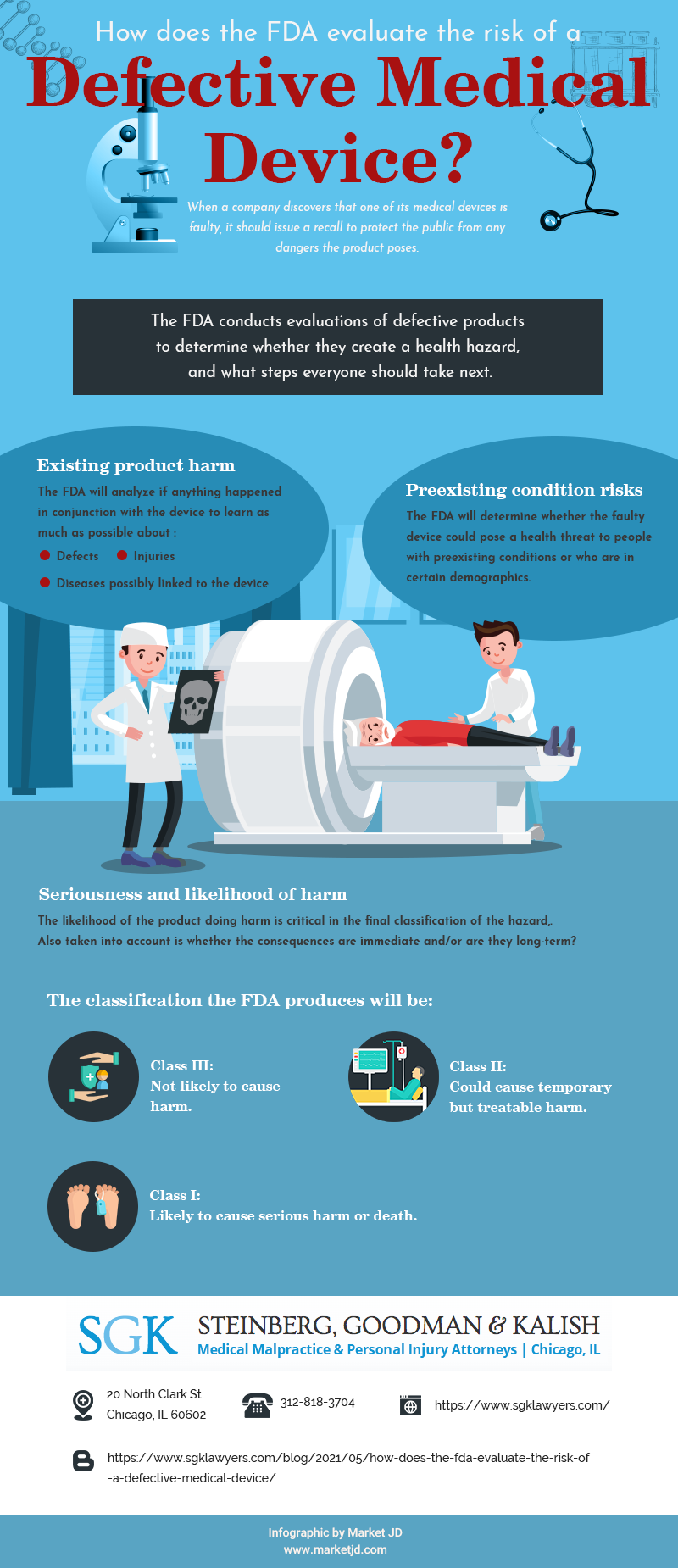
Existing product harm
Has anything already happened in conjunction with the device? The FDA will analyze these incidents to learn as much as possible about the defects, injuries, and diseases possibly linked to the device.
Preexisting condition risks
The FDA will determine whether the faulty device could pose a health threat to people with preexisting conditions or who are in certain demographics. For example, a study may reveal that people over the age of 65 are more at risk of injury if they use the product.
Seriousness and likelihood of harm
How much harm is the product likely to do? How likely is it that it will occur? The answers to these questions are critical in the final calculation that leads to the classification of the hazard, as well as the assessment of whether the consequences are immediate or will be long-term.
The classification the FDA produces will be Class III: not likely to cause harm; Class II: could cause temporary but treatable harm; or Class I: likely to cause serious harm or death. The classification directly informs the next actions of the company and the FDA in keeping the public safe.

